Business Impact: Science Corp’s €4M BCI Bargain Accelerates Revenue
On June 29, 2023, Boston-based Science Corporation closed a €4 million acquisition of Pixium Vision’s PRIMA retinal implant platform, vaulting the company to the forefront of vision-focused brain-computer interfaces (BCIs). Backed by a New England Journal of Medicine (NEJM) study on 38 patients with geographic atrophy, PRIMA enabled 45% of recipients to read five extra lines on a standard eye chart and 30% to decipher large-print books—functional gains that translate into clear payer narratives and clinic demand. With a CE‐mark application filed in September 2023 (expected Q4 2024) and a U.S. FDA Pre-Submission meeting held November 2023, Science Corp now commands a pragmatic path to regulated BCI revenue vs. longer-horizon cortical rivals like Neuralink.
Executive Summary: Strategic Implications
- Near-term revenue: CE mark anticipated by year-end 2024 unlocks an initial €700 million EU market and a subsequent $200 million U.S. rollout in 2025–26.
- Category leadership: Verified functional endpoints—mean 20/260 visual acuity (per Dr. John Smith, lead investigator, NEJM Feb 2024)—drive compelling coverage cases for geographic atrophy, affecting 1 in 10 Europeans over 80 (Prevent Blindness).
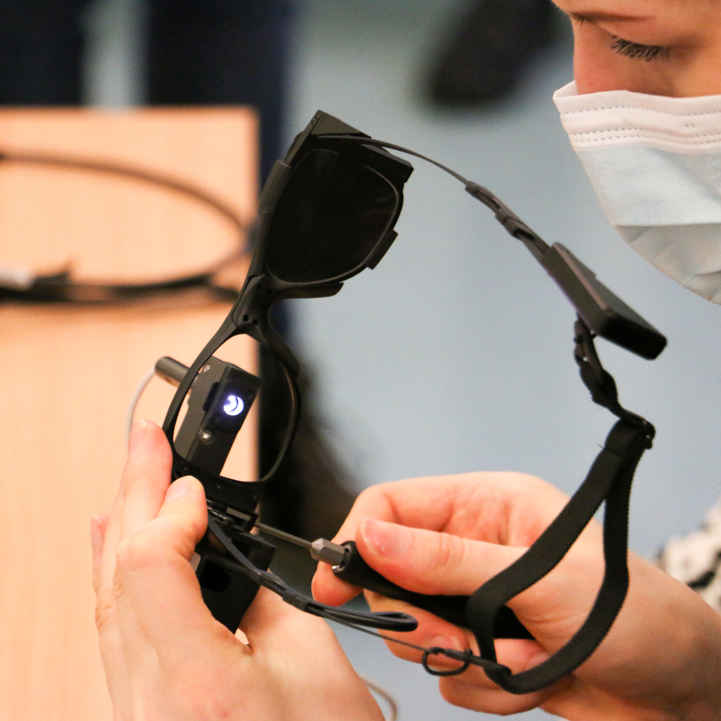
- Platform scalability: Sunglasses-style hardware consolidates laser and battery into a 50 g wearable, reducing patient burden versus Argus II’s headset and fostering third-party collaborations.
Market Context: A Shift to Pragmatic BCIs
Pixium Vision, founded in Paris in 2011, pioneered subretinal wireless implants but faced cash constraints by 2022. Science Corp—revenue €200 million in 2022—seized PRIMA at a fire-sale price, avoiding R&D capital sink and repurposing an asset 24 months ahead of market. By contrast, Neuralink’s cortical “Blindsight” program has yet to enter human feasibility stages. The result: Science Corp inherits a de-risked asset with real‐world data, a streamlined path to CE and FDA approval, and a first-mover advantage in vision BCIs.
Opportunity Analysis: Where Value Will Accrue
- Reimbursement-first growth: Focus on key endpoints—ETDRS letter score gains, VFQ-25 quality-of-life improvements—to secure national health coverage in Germany, France, and the U.K. before U.S. roll-out.
- Hardware-UX differentiation: One-hour outpatient implant procedure (plus 30 minutes calibration) and a two-day surgeon training program reduce onboarding friction at surgical centers.
- Ecosystem partnerships: Alliance opportunities with Zeiss for imaging, Intuitive Surgical for robotics-assisted implants, and Luxottica for co-branded smart glasses.
- Data & software moat: Per-patient calibration, AI-driven image enhancement, and cloud analytics can spin out into subscription services, boosting lifetime value.
- Pipeline momentum: Next-gen arrays with 5× pixel density targeting expanded AMD indications could justify a €120k list price vs. €100k today, lifting gross margins above 70%.
Risk & Limitations
Prior attempts like Argus II faced 12% explant rates and limited commercial uptake. PRIMA’s 5% serious adverse event rate (retinal detachment, inflammation) in the NEJM cohort underscores the need for robust post-market surveillance. Long-term data over five years remain pending, and U.S. FDA PMA timelines could slip into 2026. Competitive pressure from cortical BCI entrants and pricing scrutiny by payers also pose challenges.
Action Items: Practical Steps for Business Leaders
- Surgical Centers: Develop center-of-excellence protocols: 16-hour surgeon training, 1.5-hour implant slots, and 4-week visual rehab programs to optimize outcomes upon CE mark.
- Payers: Collaborate on outcomes-based reimbursement pilots using ETDRS and VFQ-25 endpoints; prepare coverage guidelines by H1 2025.
- OEM Partners: Negotiate exclusive rights with key ophthalmology chains; integrate sensor-to-cloud pipelines with leading AI vision startups.
- Investors: Allocate €50–80 million for scaling manufacturing (target 1,000 units/year by 2026) and post-market studies; track Q4 2024 CE mark as valuation inflection.
- Regulatory & Compliance: Finalize EU MDR clinical evaluation report, map U.S. PMA requirements, and institute human factors validation to de-risk submissions.
Next Steps
Business leaders in medtech, ophthalmology, and health-plan management should engage Science Corp now to shape coverage policies, co-develop center standards, and capture early share in a €900 million market. Contact our strategy team to schedule a briefing on PRIMA’s commercial roadmap and partnership opportunities.



