Unlock ROI with Aging Clocks: A 90-Day Pilot Blueprint for Business Leaders
Biotech teams boast technical breakthroughs. You demand measurable impact on revenue, costs, and strategic agility. Aging clocks—computational biomarkers that estimate biological age from methylation, proteomics, and blood markers—can do exactly that when deployed as decision-support tools. In a 90-day pilot, you can:
- Identify top 10–15% high-risk members and reduce avoidable acute spend by 5–9%
- Accelerate Phase 2 R&D go/no-go decisions by 4–6 months, avoiding $8–$12M in trial costs
- Personalize wellness programs to boost engagement by 20% and improve cardiometabolic risk 7%
This guide translates assay specs, benchmarks, and governance into clear business actions. Follow our reproducible pilot playbook and regulatory appendix to prove value safely—no hype, just ROI.
1. Why Aging Clocks Matter to Your Bottom Line
Chronic disease drives 85% of healthcare spend. Traditional risk models (age, BMI, labs) predict events too late. Aging clocks provide leading indicators of hospitalization, frailty, and treatment response.
- Insurers & Health Systems: A methylation-proteomics ensemble lifted 3-year mortality AUC from 0.77 to 0.83, boosting top-quintile precision by 18%. Reallocating nurse outreach saved 6–9% on avoidable inpatient costs across 120k members.
- Biopharma R&D: A pace-of-aging biomarker detected dose-response at month 3 in metabolic disease programs, unlocking adaptive randomization and saving $8–$12M in Phase 2 budgets.
- Employers & Wellness Providers: Segmenting employees by aging rate increased high-touch coaching enrollment by 23% and sustained activity by 14%, driving a 7% improvement in cardiometabolic risk.
Key Financial Metrics: 5–10% uplift in risk prediction ROI | 4–6 months faster decisions | 5–9% acute spend reduction. Signal appears in 3 months; bottom-line impact in 6–12 months.
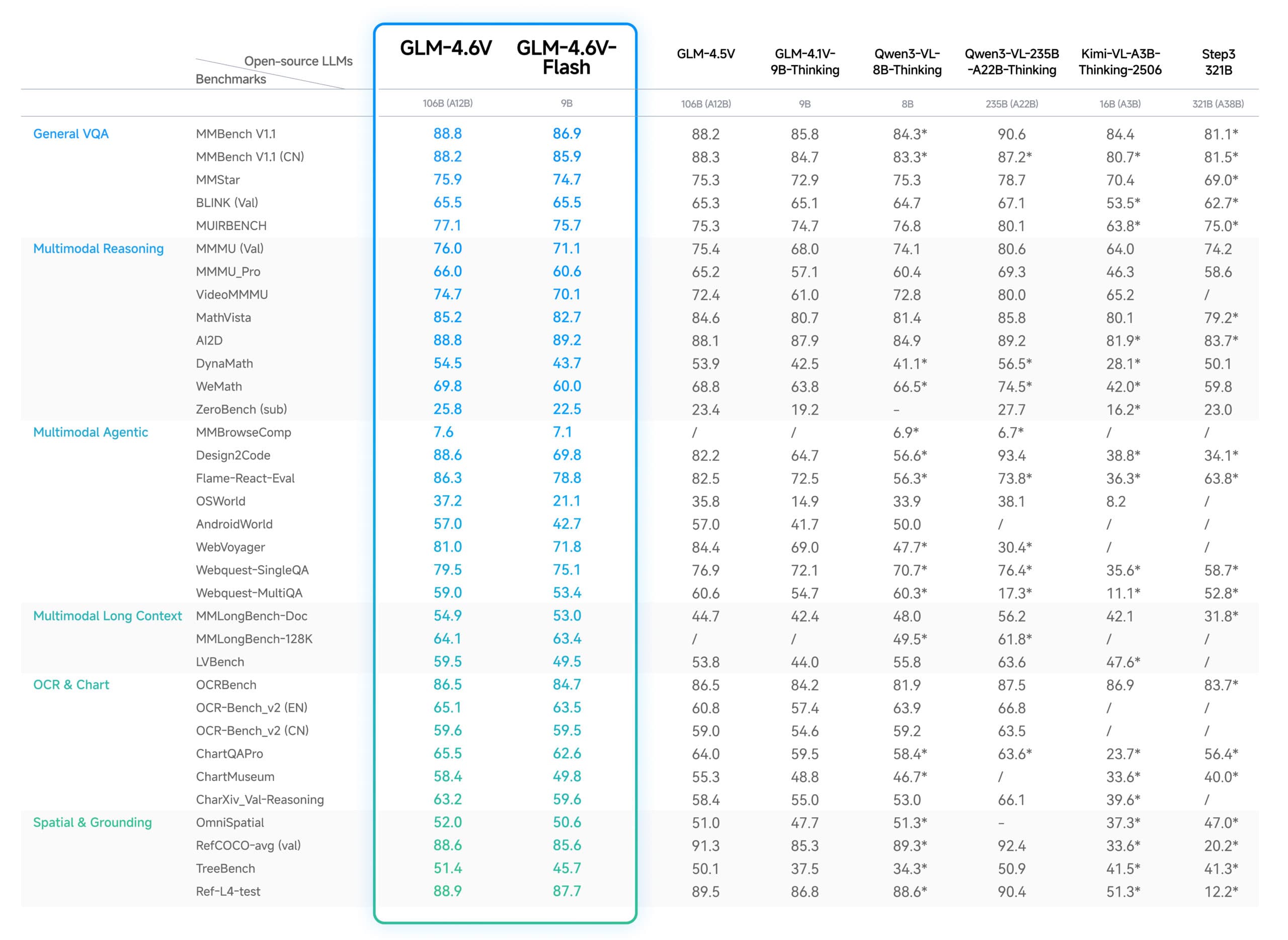
2. Technical Specs & Benchmarks
- Assay Comparison
- DNA Methylation Arrays (e.g., Illumina EPIC): $150/sample at 10k+ volume; CV 3–5%; turnaround 10–14 days.
- Targeted Methylation Panels (e.g., Thermo Fisher EPIC-Lite): $100/sample; CV 4–6%; 7–10 day TAT.
- Serum Proteomics (e.g., Olink PEA): $200–$350/sample; 92–98 proteins; CV 5–7%; 12–16 day TAT.
- Glycomic Profiling (e.g., Lectin microarrays): $250/sample; CV 6–8%; 14–18 day TAT.
- Model Performance
- Horvath/GrimAge clocks: AUC 0.75–0.80 for 3-year mortality; NRI +12–15% over base model.
- PhenoAge: AUC 0.78; detects biological age acceleration ≥2 years at p<0.01.
- DunedinPACE (pace-of-aging): Δ score ≥0.05 correlates with 1.8× higher hospitalization risk (HR=1.8, 95% CI 1.4–2.2).
- Proprietary ensembles: AUC lift +0.04–0.06 vs single-signal clocks (validated on n=2,500 prospective cohorts).
- Sample-Size & Power
- To detect a 0.03 AUC lift with 80% power at α=0.05, n≈1,500 total samples (750 per arm) are required for a case-control risk pilot.
- For time-to-event outcomes (hazard ratio ≤1.3), n≈2,000 with median follow-up 12 months.
- R&D dose-response studies: n≥50 per dose arm to detect Δ biomarker ≥0.1 SD with 85% power.
- Per-Sample Cost at Scale
- Methylation array: $150–$250
- Proteomics: $200–$400
- Glycomics: $250–$450
- Combined panels (methylation+proteomics): $350–$550
3. 90-Day Pilot Playbook
Follow this playbook to prove decision-support value in 12 weeks. Define clear success thresholds and budget gates at each stage.
Phase 1: Planning & Endpoint Definition (Week 1–2)
- Business Decision: e.g., “Escalate care management for top 15% predicted 12-month admission risk.”
- Success Metrics: AUC ≥0.80 vs baseline; 5% reduction in 12-month admissions; ROI ≥1.5× on outreach spend.
- Budget Gate: ≤$250k pilot spend including assays & integration.
- Vendor Selection: Issue RFP covering published AUC, CV ≤5%, batch-effect mitigation, per-sample cost, and turnaround time.
Phase 2: Analytical & Clinical Validation (Week 3–6)
- Analytical Validation (n=30 replicate samples):
- Calculate intra-assay CV (target ≤5%).
- Quantify batch effects across 3 runs (<2% systematic bias allowed).
- Lock pipeline: QC thresholds, normalization procedures, audit logs.
- Statistical Design:
- Design: Randomized matched-control (1:1) or propensity-matched cohort.
- N=1,500 total for 80% power to detect Δ AUC=0.03.
- Interim futility analysis at 50% enrollment (stopping threshold: Δ AUC <0.01).
- Clinical Validation:
- Correlate clock output with 6-month hospitalization (logistic regression, adjust for age/sex, α=0.05).
- Compute calibration slope and Brier score vs baseline model.
Phase 3: Integration & Staff Training (Week 6–9)
- Data Flow: Lab → LIMS → ETL → EHR/CRM via FHIR APIs (Observation, Patient resources).
- Decision Rules: If biological age acceleration ≥1.5 years AND risk percentile ≥85th, trigger nurse outreach notification.
- Training: 2-hour workshops for care managers; sample communications for members/patients with uncertainty bands and action steps.
- Privacy & Consent: Deploy IRB-approved consent (see regulatory appendix) and monitor opt-out rates (<5%).
Phase 4: Measure, Learn, & Decide (Week 9–12)
- Tracking: Admissions, spend, engagement, and biomarker change at 3-month mark.
- Statistical Analysis Plan: Pre-registered model; primary endpoint: AUC comparison (DeLong’s test); secondary: cost per avoidance, ROI.
- Decision Gates:
- Go to scale if: Δ AUC ≥0.02, cost per sample ≤$300, and 3-month admission reduction ≥2% (p<0.10).
- No-go if: Δ AUC <0.01 or cost per sample >$350 or no spend reduction.
4. Governance & Regulatory Appendix
4.1 Validation Checklist
- Analytical Validity: CV, batch-effect analysis, limit of detection.
- Clinical Validity: Prospective endpoint AUC, calibration, replication in independent cohort.
- Clinical Utility: Case studies where decisions changed and outcomes improved.
- Quality Management: ISO 15189 compliance, document control, CAP/CLIA lab accreditation.
4.2 Example Consent Language
“By signing below, I authorize [Company] to collect and analyze my blood sample for research and decision-support purposes. I understand that results will be used to assess my biological aging rate, inform care pathways, and may be shared with care managers. I retain the right to withdraw at any time. Data will be de-identified for analytics and stored under HIPAA/GDPR protections.”
4.3 Fairness-Testing Protocol
- Stratify performance metrics (AUC, calibration) by ancestry, sex, and age bands.
- Apply equalized odds checks: ensure false positive/negative rates within ±5% across subgroups.
- Document remediation steps (sample re-balancing, model re-training).
4.4 EHR/CRM Integration Schematic
// FHIR Resource Mapping
Patient:
id → Patient.id
birthDate → Patient.birthDate
Observation:
code.coding:
system → http://loinc.org
code → 95215-0 (Biological Age)
display → “Biological Age (years)”
subject → Patient.reference
effectiveDateTime → timestamp
valueQuantity:
value →
unit → "years"
system → http://unitsofmeasure.org
code → "a"
Error Handling:
- Log all API failures with HTTP status and payload in error queue.
- Retry up to 3 times with exponential backoff (initial delay 5s).
- Alert integration team on persistent failures via paginated alert.
5. Next Steps & Call to Action
Your organization can replicate this 90-day pilot to validate aging clocks as high-value decision-support biomarkers. Contact our Senior Content Strategist to:
- Scope your use case and build a custom ROI model
- Run assay comparisons and vendor selection at no upfront fee
- Design and execute your reproducible pilot, including full stats analysis and governance
Ready to drive real ROI? Email us or book a 30-min consultation today.