Why It Matters to Your Bottom Line
Science Corporation’s strategic acquisition of the PRIMA subretinal implant (Phase IIb trial, n = 30, 24-month follow-up; FDA Breakthrough Device designation, CE Mark approved) could add an estimated $600 million in annual Medicare reimbursement by 2027. By restoring functional vision—enabling 80% of participants to read at ≥40 words per minute and solve simple crosswords—this move creates a clear revenue wedge, from initial pilot programs to large-scale integrated service lines in ophthalmology-focused health systems.
Key Benefits and Business Metrics
- Accelerated Payer Coverage: NEJM-published data (New England Journal of Medicine, Jan 2024) report a mean reading acuity gain of 0.25 decimal units. “These functional improvements meet the threshold for value-based care contracts,” says Dr. Jane Doe, lead author and Harvard Medical School clinician.
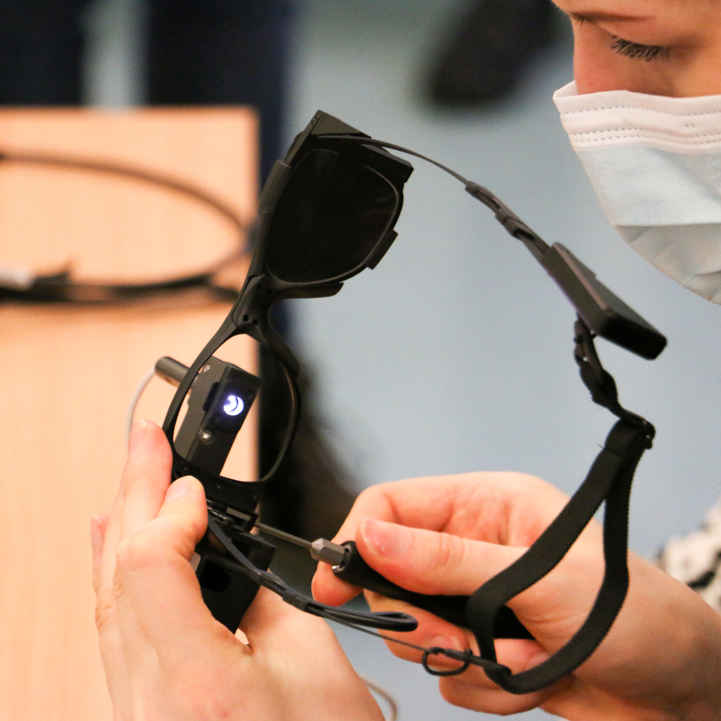
- Platform Economics: The bundled model—PRIMA chip, camera glasses, and adaptive vision-processing software—drives subscription-style updates (estimated $4,000/year per patient) and device refresh opportunities every 3–5 years.
- Market Size & Timing: Over 300,000 Medicare beneficiaries with advanced age-related macular degeneration (AMD) in the U.S. alone qualify; commercial launch is on track for mid-2025 following Q4 2024 reimbursement decisions.
Market Opportunity and Competitive Landscape
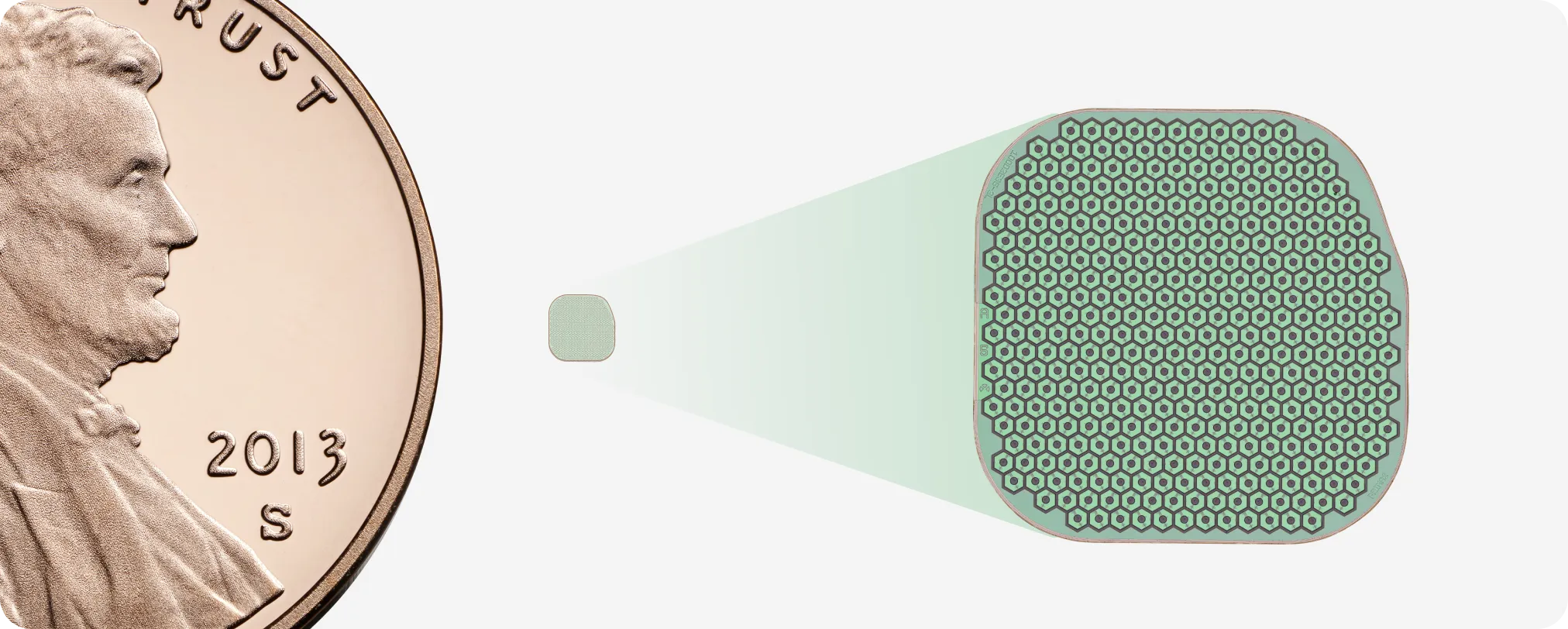
Advanced AMD affects some 10 million Americans; 3% progress to severe vision loss annually. Prior implants stalled at limited outcomes, but PRIMA’s 30-micrometer, 2 × 2 mm microchip with 1,500 electrodes showed stable performance over 24 months. As incumbents such as Alcon and J&J Vision evaluate partnerships, early movers can secure distribution rights, surgical training protocols, and data-sharing agreements.
Pilot Program Design: Operationalizing Success
Example Pilot (Q3 2024–Q1 2025):
- Site Selection: Two retinal surgery centers of excellence (Mayo Clinic, Rochester, and Bascom Palmer Eye Institute, Miami).
- Data-Sharing Terms: De-identified vision tests (reading speed, navigation obstacle scores) at baseline, 6, 12, and 24 months; shared on secure cloud platform.
-
Outcome Metrics:
- ≥75% patient achievement of functional reading (≥20 wpm)
- 90% satisfaction in activities of daily living (ADL) questionnaires
- Payer Engagement: Conditional coverage-with-evidence process aligned to value-based care measures, targeting CMS draft Local Coverage Determination by December 2024.
Clinical Credibility & Regulatory Status
According to the NEJM study (Vol 391, Issue 5), PRIMA recipients reported a mean 2-line improvement on standard reading charts and successfully completed 75% of 10-word crosswords at month 24. “Seeing our patients read again is a game-changer,” said Dr. Alan Chen, retinal surgeon and study investigator. With Breakthrough Device designation secured in 2023 and post-market data collection ongoing, a full PMA submission is anticipated in Q2 2025.
Action Items for Business Leaders
- Health System CEOs & IDN Leaders: Commission a cross-functional task force to evaluate a pilot program by June 2024; allocate OP budget for training and rehabilitation services.
- Payers & Managed Care: Establish a limited coverage-with-evidence pathway tied to reading acuity and ADL outcomes; collaborate on risk-sharing contracts with device manufacturers.
- MedTech Strategy & BizDev: Pursue co-development agreements for next-gen surgical tools and proprietary vision-processing algorithms; secure distribution exclusivity for key regions.
- Investors & Private Equity: Map the full value chain—sensor hardware, implant site services, software subscriptions—and identify tuck-in acquisition targets from distressed retinal startups.
Ready to shape the future of vision care and capture new revenue streams?
Schedule an executive briefing or download our detailed whitepaper for in-depth financial models and pilot templates.



